PIPELINE
Our Pipeline is comprised of Biologics, including the proprietary BEAT® MultispecificsTM platform, and one Cbl-b inhibitor small molecule targeting the spectrum of hematological cancers and solid tumors. If you are interested in exploring a partnership, Contact us.
Oncology
- Overview
- ISB 2001
| ASSETS | DESCRIPTION | INDICATION |
|---|---|---|
| CLINICAL ASSETS |
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
ISB 2001
BCMA x CD38 x CD3 TREAT ™
trispecific T-Cell Engager
Multiple Myeloma
PHASE 1
ORPHAN DRUG
GRC 65327
Cbl-b Inhibitor Small Molecule
Solid Tumors
PRE-CLINICAL
CANDIDATES
ISB 2301
IMMUNITE™
NK-Cell Engager
Solid Tumors
DISCOVERY
PRODUCTS
COMPOUND
CLINICAL ASSETS
ISB 2001
TARGET
BCMA x CD38 x CD3 TREAT ™
trispecific T-Cell Engager
INDICATION
Multiple Myeloma
PHASE
STATUS :
PHASE 1 ORPHAN DRUG
PRODUCTS
COMPOUND
CLINICAL ASSETS
GRC 65327
TARGET
Cbl-b Inhibitor Small Molecule
INDICATION
Solid Tumors
PHASE
STATUS :
PHASE 1
PRODUCTS
COMPOUND
CANDIDATES
ISB 2301
TARGET
IMMUNITE™
NK-Cell Engager
INDICATION
Solid Tumors
PHASE
STATUS :
DISCOVERY
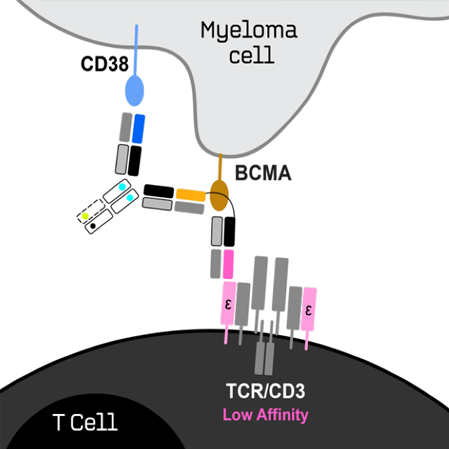
ISB 2001 is first TREATTM Trispecific Antibody for Relapsed/Refractory Multiple Myeloma
KEY ATTRIBUTES
- BCMA and CD38 are expressed on the surface of multiple myeloma cells
and are clinically validated targets.
- ISB 2001 combines three proprietary Fab arms binding to CD3 on T-cells, and to BCMA and CD38 on myeloma cells.
- In vitro studies showed increased killing potency of tumor cells compared to all tested antibodies, including currently approved and investigational multiple myeloma therapies.
- In vivo studies in multiple myeloma models also show superior potency relative to antibodies for the treatment of multiple myeloma.
- ISB 2001 redirects CD3+ T lymphocytes to kill tumor cells expressing from low
to high levels of both BCMA and CD38. - With two different tumor-associated antigens, ISB 2001 is expected to be more resistant to antigen escape associated with treatment of MM patients.
- Ichnos received authorizations from HREC in Australia and the U.S. FDA to initiate a Phase 1 first-in-human study of ISB 2001 for the treatment of MM and was granted ODD by the U.S. FDA for the same indication.
TREAT™: Trispecific Engagement by Antibodies based on the TCR MHC:
Major histocompatibility complex, CDC: Complement-Dependent
Cytotoxicity ADCC: Antibody-Dependent Cell-mediated Cytotoxicity
For collaborations, please contact us here.
Inflammation and Autoimmune Disease
The autoimmune disease assets have been out-licensed to enable greater focus on oncology. Explore the pipeline chart below to learn more and
Contact Us for additional information.
Autoimmune Disease
PRODUCTS
Telazorlimab (and ISB 830-X8)
DESCRIPTION
OX40 antagonist
Monoclonal Antibody
Atopic Dermatitis*
| PRODUCTS | DESCRIPTION | |
|---|---|---|
| PRODUCTS |
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
Licensed to
$320 million for upfront payment, development, regulatory and sales milestone payments,
plus tiered royalties on global sales
Telazorlimab
Atopic Dermatitis*
SUCCESSFUL
PHASE 2B*
Licensed to

€20.8 million for upfront payment. Plus development, regulatory and sales milestone payments,
and tiered royalties on global sales
Partnering-Ready Assets to Accelerate Short-Term Value Creation
PRODUCTS
ASSETS
CLINICAL ASSETS
ISB 1442
DESCRIPTION
CD38 biparatopic x CD47 BEAT® Myeloid-Cell Engager
INDICATION
Multiple Myeloma; AML planned
PHASE
STATUS :
PHASE 1 ORPHAN DRUG
| ASSETS | DESCRIPTION | INDICATION |
|---|---|---|
| CLINICAL ASSETS |
| PRECLINICAL | PHASE 1 | PHASE 2 | PHASE 3 |
|---|---|---|---|
| STATUS |
|---|
ISB 1442
CD38 biparatopic x CD47 BEAT® Myeloid-Cell Engager
Multiple Myeloma
PHASE 1
ORPHAN DRUG
